×
![]()
Chemical Molecules
Molecule Details
| Molecule ID | MOL9 | ||
| Compound Name | androsta-4,16-dien-3-one | ||
| ⤓Download 2D Structure File | ⤓Download 3D Structure File | View Structure Image |
Structure Image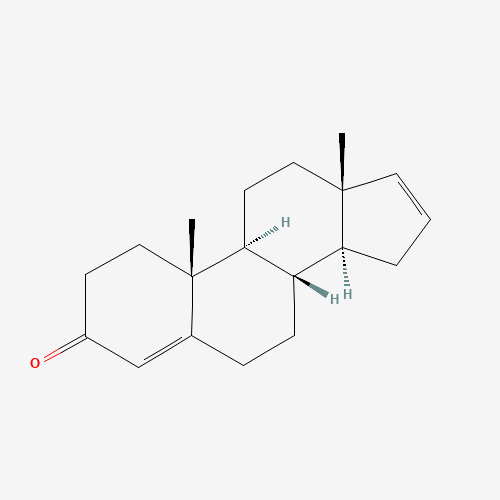
|
Primary Details
| Compound Name | androsta-4,16-dien-3-one |
| Synonym Name | Androstadienone, 4,16-Androstadien-3-one, 4075-07-4, ZUZ4FHD36E |
| CAS ID | 7/4/4075 |
| IUPAC Name | (8S,9S,10R,13R,14S)-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15-decahydrocyclopenta[a]phenanthren-3-one |
| Molecular Formula | C19H26O |
| Chemical Safety | Nil |
| External Database ID |
92979 |
| Inchi | InChI=1S/C19H26O/c1-18-9-3-4-16(18)15-6-5-13-12-14(20)7-11-19(13,2)17(15)8-10-18/h3,9,12,15-17H,4-8,10-11H2,1-2H3/t15-,16-,17-,18-,19-/m0/s1 |
| INCHI Key | HNDHDMOSWUAEAW-VMXHOPILSA-N |
| Canonical SMILES | CC12CCC3C(C1CC=C2)CCC4=CC(=O)CCC34C |
| Molecule Type | Natural |
| Group | Phytochemicals |
| Sub-Group 1 | - |
| Sub-Group 2 | - |
| Sub-Group 3 | - |
| Sub-Group 4 | - |
| Sub-Group 5 | - |
| Description |
Androstadienone, also known as androsta-4,16-dien-3-one, belongs to the 16-androstene class of endogenous steroids and has been identified for its significant pheromone-like effects in humans. This compound is produced from androstadienol through the action of 3β-hydroxysteroid dehydrogenase. Furthermore, it can undergo conversion into androstenone, a more potent and fragrant pheromone, by the enzyme 5α-reductase. Subsequently, 5α-reductase can further transform androstenone into 3α-androstenol or 3β-androstenol, both of which are also more potent and aromatic pheromones, through the activity of 3-ketosteroid reductase. |
Chemical & Physical Properties
| Molecular Weight | 270.4 g/mol |
| LogP (Octanol-Water) | 4.421 |
| Hydrogen Donor Count | 0 |
| Bond Acceptor Count | 1 |
| Rotable Bond Count | 0 |
| Topological Surface Area | 17.07 Ų |
| Heavy Atom Count | |
| Melting Point | Nil |
| Boiling Point | Nil |
| Water Solubility | Nil |
| Henry's Law Constant | Nil |
| pKa Dissociation Constant | Nil |
| Vapour Pressure | Nil |
| Molecule Density | Nil |
| Molecule Stability | Nil |
| Kovats retention Index | Nil |
| Physical Description |
|
Spectra Information
Biological Activities
| Activity Name | Nil |
| Molecule Target | Nil |
| Comment |
|
| References |
|




