Chemical Molecules
Molecule Details
| Molecule ID | MOL87 | ||
| Compound Name | Bicyclogermacrene | ||
| ⤓Download 2D Structure File | ⤓Download 3D Structure File | View Structure Image |
Structure Image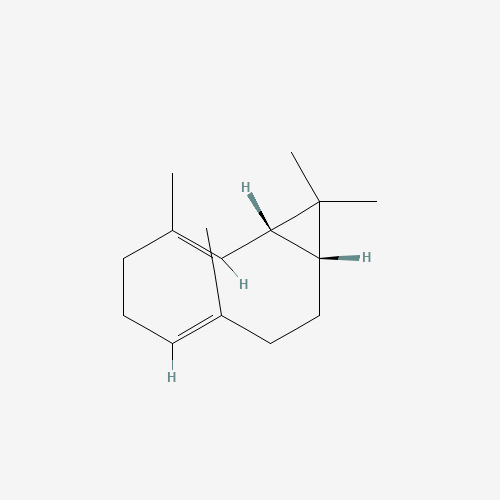
|
Primary Details
| Compound Name | Bicyclogermacrene |
| Synonym Name | Bicyclogermacrene, CHEBI:63709, (1S,2E,6E,10R)-3,7,11,11-tetramethylbicyclo[8.1.0]undeca-2,6-diene, LMPR0103100001, C20175 |
| CAS ID | 24703-35-3 |
| IUPAC Name | (1S,2E,6E,10R)-3,7,11,11-tetramethylbicyclo[8.1.0]undeca-2,6-diene |
| Molecular Formula | C15H24 |
| Chemical Safety | |
| External Database ID |
13894537 |
| Inchi | InChI=1S/C15H24/c1-11-6-5-7-12(2)10-14-13(9-8-11)15(14,3)4/h6,10,13-14H,5,7-9H2,1-4H3/b11-6+,12-10+/t13-,14+/m1/s1 |
| INCHI Key | VPDZRSSKICPUEY-JEPMYXAXSA-N |
| Canonical SMILES | CC1=CCCC(=CC2C(C2(C)C)CC1)C |
| Molecule Type | Natural |
| Group | Phytochemicals |
| Sub-Group 1 | Terpenoids (Isoprenoids) |
| Sub-Group 2 | sesquiterpenoid |
| Sub-Group 3 | - |
| Sub-Group 4 | - |
| Sub-Group 5 | - |
| Description |
Sesquiterpene bicyclogermacrene is obtained from germacrane by cyclization across the C(8)-C(9) link and dehydrogenation across the C(1)-C(10) and C(4)-C(5) bonds. It performs a metabolite function. It comes from a germacrane hydride. |
Chemical & Physical Properties
| Molecular Weight | 204.35Â g/mol |
| LogP (Octanol-Water) | 4.1 |
| Hydrogen Donor Count | 0 |
| Bond Acceptor Count | 0 |
| Rotable Bond Count | 0 |
| Topological Surface Area | 0 |
| Heavy Atom Count | 15 |
| Melting Point | |
| Boiling Point | |
| Water Solubility | |
| Henry's Law Constant | |
| pKa Dissociation Constant | |
| Vapour Pressure | |
| Molecule Density | |
| Molecule Stability | |
| Kovats retention Index | |
| Physical Description |
|
Spectra Information
Biological Activities
| Activity Name | antimicrobial activity and Antifungal activity |
| Molecule Target | |
| Comment |
|
| References |
1. Xiong, G., Wu, Z., Yi, J., Fu, L., Yang, Z., Hsieh, C., ... & Cao, D. (2021). ADMETlab 2.0: an integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic acids research, 49(W1), W5-W14. 2. Kim, S., Chen, J., Cheng, T., Gindulyte, A., He, J., He, S., ... & Bolton, E. E. (2019). PubChem 2019 update: improved access to chemical data. Nucleic acids research, 47(D1), D1102-D1109. 3. Valarezo, E., Flores-Maza, P., Cartuche, L., Ojeda-Riascos, S., & RamÃrez, J. (2021). Phytochemical profile, antimicrobial and antioxidant activities of essential oil extracted from Ecuadorian species Piper ecuadorense sodiro. Natural product research, 35(24), 6014–6019. https://doi.org/10.1080/14786419.2020.1813138 |




