×
![]()
Chemical Molecules
Molecule Details
| Molecule ID | MOL8 | ||
| Compound Name | caryophelline oxide | ||
| ⤓Download 2D Structure File | ⤓Download 3D Structure File | View Structure Image |
Structure Image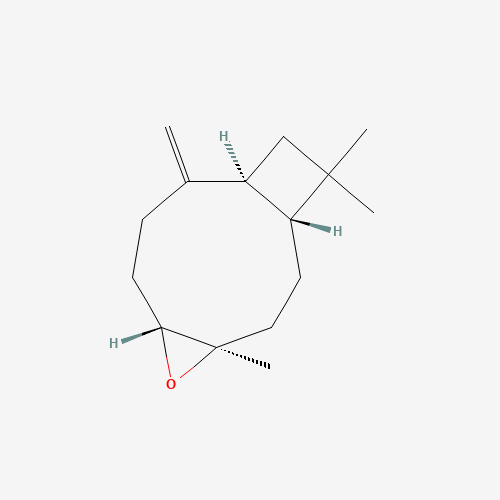
|
Primary Details
| Compound Name | caryophelline oxide |
| Synonym Name | (-)-Caryophyllene oxide, 1139-30-6, beta-Caryophyllene oxide, and beta-Caryophyllene epoxide |
| CAS ID | 1139-30-6 |
| IUPAC Name | (1R,4R,6R,10S)-4,12,12-trimethyl-9-methylidene-5-oxatricyclo[8.2.0.04,6]dodecane |
| Molecular Formula | C15H24O |
| Chemical Safety | Irritant |
| External Database ID | 1742210 |
| Inchi | InChI=1S/C15H24O/c1-10-5-6-13-15(4,16-13)8-7-12-11(10)9-14(12,2)3/h11-13H,1,5-9H2,2-4H3/t11-,12-,13-,15-/m1/s1 |
| INCHI Key | NVEQFIOZRFFVFW-RGCMKSIDSA-N |
| Canonical SMILES | CC1(CC2C1CCC3(C(O3)CCC2=C)C)C |
| Molecule Type | Natural |
| Group | Phytochemicals |
| Sub-Group 1 | Terpenoids (Isoprenoids) |
| Sub-Group 2 | sesquiterpenoid |
| Sub-Group 3 | - |
| Sub-Group 4 | - |
| Sub-Group 5 | - |
| Description |
Caryophyllene, formally known as (−)-β-caryophyllene (BCP), is a naturally occurring bicyclic sesquiterpene present in various essential oils, including but not limited to clove oil, derived from the stems and flowers of Syzygium aromaticum (cloves), the essential oil of Cannabis sativa, copaiba, rosemary, and hops. Typically, it is found in combination with isocaryophyllene (the cis double bond isomer) and α-humulene (formerly referred to as α-caryophyllene), which is a ring-opened isomer. Caryophyllene is distinguished by its cyclobutane ring and a trans-double bond within a 9-membered ring, both of which are uncommon features in the natural world. |
Chemical & Physical Properties
| Molecular Weight | 220.35 g/mol |
| LogP (Octanol-Water) | 4.474 |
| Hydrogen Donor Count | 1 |
| Bond Acceptor Count | 0 |
| Rotable Bond Count | 0 |
| Topological Surface Area | 12.53 Ų |
| Heavy Atom Count | 16 |
| Melting Point | Nil |
| Boiling Point | Nil |
| Water Solubility | Nil |
| Henry's Law Constant | Nil |
| pKa Dissociation Constant | Nil |
| Vapour Pressure | Nil |
| Molecule Density | Nil |
| Molecule Stability | Nil |
| Kovats retention Index | Nil |
| Physical Description |
|
Spectra Information
Biological Activities
| Activity Name | Anticancer, Antioxidant, and Antimicrobial activity |
| Molecule Target | multiple cellular targets |
| Comment |
|
| References |
|




