×
![]()
Chemical Molecules
Molecule Details
| Molecule ID | MOL56 | ||
| Compound Name | gamma-elemene | ||
| ⤓Download 2D Structure File | ⤓Download 3D Structure File | View Structure Image |
Structure Image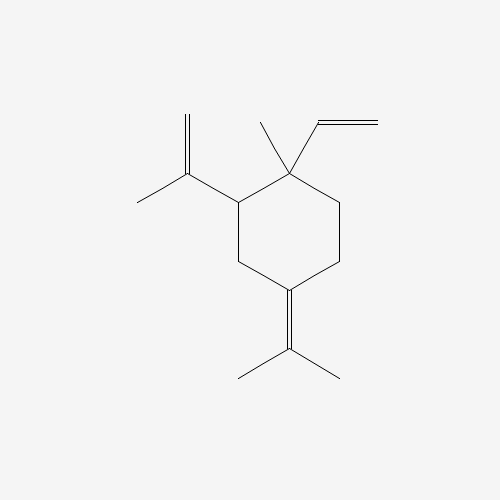
|
Primary Details
| Compound Name | gamma-elemene |
| Synonym Name | (-)-.gamma.-Elemene, DTXSID70954182 |
| CAS ID | 3242-08-8 |
| IUPAC Name | 1-ethenyl-1-methyl-4-propan-2-ylidene-2-prop-1-en-2-ylcyclohexane |
| Molecular Formula | C15H24 |
| Chemical Safety | |
| External Database ID |
94254 |
| Inchi | InChI=1S/C15H24/c1-7-15(6)9-8-13(11(2)3)10-14(15)12(4)5/h7,14H,1,4,8-10H2,2-3,5-6H3 |
| INCHI Key | BQSLMQNYHVFRDT-UHFFFAOYSA-N |
| Canonical SMILES | CC(=C1CCC(C(C1)C(=C)C)(C)C=C)C |
| Molecule Type | Natural |
| Group | Phytochemicals |
| Sub-Group 1 | Terpenoids (Isoprenoids) |
| Sub-Group 2 | sesquiterpenoid |
| Sub-Group 3 | - |
| Sub-Group 4 | - |
| Sub-Group 5 | - |
| Description |
Elemenes form a closely associated set of naturally occurring chemical compounds present in various plants. Consisting of α-, β-, γ-, and δ-elemene, these compounds are structural isomers of one another and are categorized as sesquiterpenes. |
Chemical & Physical Properties
| Molecular Weight | 204.35 g/mol |
| LogP (Octanol-Water) | 4.987 |
| Hydrogen Donor Count | 0 |
| Bond Acceptor Count | 0 |
| Rotable Bond Count | 2 |
| Topological Surface Area | 0 |
| Heavy Atom Count | 15 |
| Melting Point | Nil |
| Boiling Point | Nil |
| Water Solubility | Nil |
| Henry's Law Constant | Nil |
| pKa Dissociation Constant | Nil |
| Vapour Pressure | Nil |
| Molecule Density | Nil |
| Molecule Stability | Nil |
| Kovats retention Index | Standard polar: 1636, 1607, 1644, 1636, 1633, 1644, 1633, 1641, 1650, 1639, 1663, 1633, 1614, 1625, 1608, 1609, 1658, 1650, 1618, 1641, 1650, 1650, 1650, 1650, 1650, 1650, 1624, 1650, 1636, 1618, 1626, 1639, 1636, 1638, 1650, 1650, 1650, 1636, 1641, 1619, 1643.2, 1650, 1642, 1650, 1625, 1647, 1628, 1651, 1620, 1651, 1651, 1665, 1648, 1625, 1642, 1642, 1514 |
| Physical Description |
|
Spectra Information
Biological Activities
| Activity Name | antileishmanial activity |
| Molecule Target | |
| Comment |
|
| References |
|




