×
![]()
Chemical Molecules
Molecule Details
| Molecule ID | MOL35 | ||
| Compound Name | E-isolongifolanone | ||
| ⤓Download 2D Structure File | ⤓Download 3D Structure File | View Structure Image |
Structure Image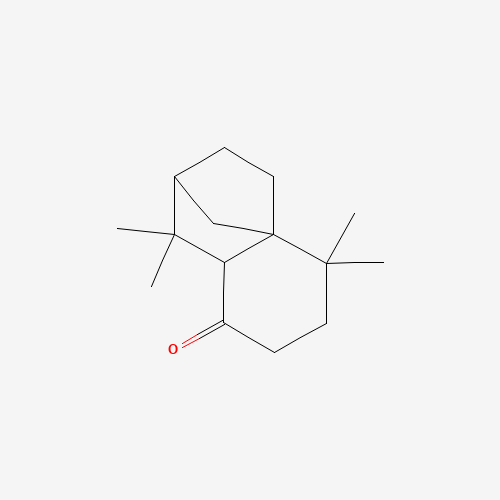
|
Primary Details
| Compound Name | E-isolongifolanone |
| Synonym Name | Isolongifolone |
| CAS ID | 23787-90-8 |
| IUPAC Name | 2,2,8,8-tetramethyl-octahydro-1H-2,4a-methanonapthalen-10-one |
| Molecular Formula | C15H24O |
| Chemical Safety | Irritant, Enviornmental Hezard |
| External Database ID |
90978 |
| Inchi | InChI=1S/C15H24O/c1-13(2)7-6-11(16)12-14(3,4)10-5-8-15(12,13)9-10/h10,12H,5-9H2,1-4H3 |
| INCHI Key | VCOCESNMLNDPLX-UHFFFAOYSA-N |
| Canonical SMILES | CC1(CCC(=O)C2C13CCC(C3)C2(C)C)C |
| Molecule Type | Natural |
| Group | Phytochemicals |
| Sub-Group 1 | Terpenoids (Isoprenoids) |
| Sub-Group 2 | sesquiterpenoid |
| Sub-Group 3 | - |
| Sub-Group 4 | - |
| Sub-Group 5 | - |
| Description |
Isolongifolone is a clear liquid characterized by a dry, woody aroma reminiscent of earthy patchouli with camphoraceous notes. It is produced through the oxidation of isolongifolene using peroxide under acidic conditions, such as hydrogen peroxide in formic acid. The primary application of this substance is in the formulation of perfume oils for use in soaps and fabric detergents. |
Chemical & Physical Properties
| Molecular Weight | 220.35 g/mol |
| LogP (Octanol-Water) | 4.155 |
| Hydrogen Donor Count | 0 |
| Bond Acceptor Count | 1 |
| Rotable Bond Count | 0 |
| Topological Surface Area | 17.1 Ų |
| Heavy Atom Count | 16 |
| Melting Point | Nil |
| Boiling Point | Nil |
| Water Solubility | Nil |
| Henry's Law Constant | Nil |
| pKa Dissociation Constant | Nil |
| Vapour Pressure | Nil |
| Molecule Density | Nil |
| Molecule Stability | Nil |
| Kovats retention Index | Semi-standard non-polar: 1606, 1627, 1611, 1610, 1616, 1606, 1613, 1618, 1620, 1619, 1592 |
| Physical Description |
|
Spectra Information
Biological Activities
| Activity Name | antioxidant, anti-inflammatory, anticancer, and neuroprotective |
| Molecule Target | |
| Comment |
|
| References |
|




