×
![]()
Chemical Molecules
Molecule Details
| Molecule ID | MOL32 | ||
| Compound Name | Hinesol | ||
| ⤓Download 2D Structure File | ⤓Download 3D Structure File | View Structure Image |
Structure Image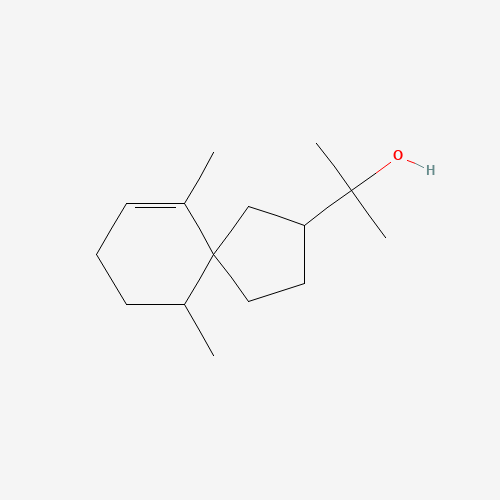
|
Primary Details
| Compound Name | Hinesol |
| Synonym Name | Agaruspirol |
| CAS ID | 59331-07-6 |
| IUPAC Name | 2-(6,10-dimethylspiro[4.5]dec-9-en-3-yl)propan-2-ol |
| Molecular Formula | C15H26O |
| Chemical Safety | Nil |
| External Database ID |
289964 |
| Inchi | InChI=1S/C15H26O/c1-11-6-5-7-12(2)15(11)9-8-13(10-15)14(3,4)16/h6,12-13,16H,5,7-10H2,1-4H3 |
| INCHI Key | ICWHTQRTTHCUHW-UHFFFAOYSA-N |
| Canonical SMILES | CC1CCC=C(C12CCC(C2)C(C)(C)O)C |
| Molecule Type | Natural |
| Group | Phytochemicals |
| Sub-Group 1 | Terpenoids (Isoprenoids) |
| Sub-Group 2 | sesquiterpenoid |
| Sub-Group 3 | - |
| Sub-Group 4 | - |
| Sub-Group 5 | - |
| Description |
Hinesol is present in diverse essential oils, exhibiting antifungal properties in oils sourced from agarwood of Aquilaria sinensis, antimicrobial effects in oils derived from the Phebalium squamulosum species complex, anti-inflammatory attributes in oils extracted from agarwood of Aquilaria agallocha, and anticancer potential in oils obtained from Cedrelopsis grevei leaves. Nevertheless, there is no documented evidence attributing these various activities to Hinesol. |
Chemical & Physical Properties
| Molecular Weight | 222.37 g/mol |
| LogP (Octanol-Water) | 4.107 |
| Hydrogen Donor Count | 1 |
| Bond Acceptor Count | 1 |
| Rotable Bond Count | 1 |
| Topological Surface Area | 20.2 Ų |
| Heavy Atom Count | 16 |
| Melting Point | Nil |
| Boiling Point | Nil |
| Water Solubility | Nil |
| Henry's Law Constant | Nil |
| pKa Dissociation Constant | Nil |
| Vapour Pressure | Nil |
| Molecule Density | Nil |
| Molecule Stability | Nil |
| Kovats retention Index | Nil |
| Physical Description |
|
Spectra Information
Biological Activities
| Activity Name | anti-cancer |
| Molecule Target | |
| Comment |
|
| References |
|




