×
![]()
Chemical Molecules
Molecule Details
| Molecule ID | MOL12 | ||
| Compound Name | solavetivone | ||
| ⤓Download 2D Structure File | ⤓Download 3D Structure File | View Structure Image |
Structure Image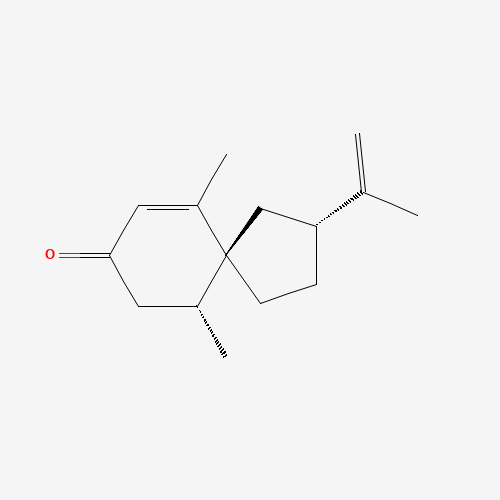
|
Primary Details
| Compound Name | solavetivone |
| Synonym Name | Katahdinone, (-)-Solavetivone, 54878-25-0, HS8O8A7REZ |
| CAS ID | 54878-25-0 |
| IUPAC Name | (3R,5S,6R)-6,10-dimethyl-3-prop-1-en-2-ylspiro[4.5]dec-9-en-8-one |
| Molecular Formula | |
| Chemical Safety | Nil |
| External Database ID |
442399Â |
| Inchi | InChI=1S/C15H22O/c1-10(2)13-5-6-15(9-13)11(3)7-14(16)8-12(15)4/h7,12-13H,1,5-6,8-9H2,2-4H3/t12-,13-,15-/m1/s1 |
| INCHI Key | FGCUSSRGQNHZRW-UMVBOHGHSA-N |
| Canonical SMILES | CC1CC(=O)C=C(C12CCC(C2)C(=C)C)C |
| Molecule Type | Natural |
| Group | Phytochemicals |
| Sub-Group 1 | Terpenoids (Isoprenoids) |
| Sub-Group 2 | sesquiterpenoid |
| Sub-Group 3 | - |
| Sub-Group 4 | - |
| Sub-Group 5 | - |
| Description |
Solavetivone, a cyclic ketone, originates from spiro[4.5]dec-6-en-8-one through the replacement of hydrogen atoms with methyl groups at positions 6 and 10, along with an isopropenyl group at position 2 (specifically the (2R,5S,10R)-diastereoisomer). Functioning as a phytoalexin and a plant metabolite, it belongs to the spiro compound category, characterized as a sesquiterpenoid and a cyclic ketone. |
Chemical & Physical Properties
| Molecular Weight | 218.33 g/mol |
| LogP (Octanol-Water) | 3.513 |
| Hydrogen Donor Count | 0 |
| Bond Acceptor Count | 1 |
| Rotable Bond Count | 1 |
| Topological Surface Area | 17.07 Ų |
| Heavy Atom Count | 16 |
| Melting Point | Nil |
| Boiling Point | Nil |
| Water Solubility | Nil |
| Henry's Law Constant | Nil |
| pKa Dissociation Constant | Nil |
| Vapour Pressure | Nil |
| Molecule Density | Nil |
| Molecule Stability | Nil |
| Kovats retention Index | Nil |
| Physical Description |
|
Spectra Information
Biological Activities
| Activity Name | Nil |
| Molecule Target | Nil |
| Comment |
|
| References |
|




